Bcl3 Shape Diagram
AX 3 has trigonal planar shape. The extra unhybridized p orbital is empty but its presence is what keeps the three sp2 orbitals separated by exactly 120 degrees.

How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube
It finds use in various applications including the production of elemental Boron.

Bcl3 shape diagram. A domain is a region in space where electrons are concentrated. However we will discuss how the hybridization occurs in detail below. They could be a lone pair a single double.
The Lewis diagram for BCl3 is. BCl3 Lewis Structure Molecular Geometry Hybridization and Shape. Draw the Lewis structure for eqBCl_3 eq.
Its shape is traigonal planar. There are lone pair s around the central atom so the geometry of BCl3 is. Molecular Orbital Diagram of BCl3.
In BCl 3 molecule boron will be the central atom which contains three bonded atoms but no lone pair of electrons. The molecular geometry of BCl3 is trigonal pyramidal. For the above molecule VSEPR notation will be AX 3 E 0.
E Number of lone pairs on central atom. Check if the molecule is heteronuclear or homonuclear. Molecular geometry and molecular shape will be the same for the molecules with 0 lone pair.
It is a colorless inorganic compound that has a pungent odor and appears as fumes in air. Use lewis structure guidelines to draw the lewis structure of BCl 3. The electron-pair geometry around the B atom in BCl3 is.
Translocation t 1419 q32q131 with immunoglobulin gene regions. Apply VSEPR notation A X E. MO diagram depicts chemical and physical traits of a molecule like bond length bond energy bond angle shape etc.
Draw the Lewis dot structure for mathBCl_3math. Boron experiences sp 2 hybridization by using a 2s and two 2p orbitals in the excitation state to give three half-filled sp 2 hybrid orbitals which are oriented in. The candidate proto-oncogene bcl-3 is related to genes.
A chromosomal aberration involving BCL3 may be a cause of B-cell chronic lymphocytic leukemia B-CLL. Lets look into the MO diagram of boron trichloride. Signs and symptoms of acute ingestion of boron trichloride may be severe and include salivation intense thirst difficulty in swallowing chills pain and shock.
Following are the steps to design the MO diagram of PCl5. BCl3 is a sp2 hyrbirdised molecule. In PCl5 it is 5 for P and 7 for every 5 atoms of Cl.
Which is the case of BCl3. This is a compound where back bonding takes place. Its steric number is also said to be 3.
Hybridization of BCl3 Boron Trichloride The type of hybridization that occurs in BCl 3 is sp 2 hybridization. However to account for the trigonal planar shape of this BCl 3 molecule sp 2 hybridization before the bond structure was advocated. This is a trigonal planar arrangement and implies that the boron must be sp2 hybridized.
ANumber of central atoms. The Lewis Structure of Boron Trichloride BCl3 has three chlorine atoms surrounding a single boron atom. XNumber of surrounding atoms.
Identify the valence electrons of each atom. Notice that the Boron has only three domains of electrons single bonds in this case. Signs and Symptoms of Acute Boron Trichloride Exposure.
The boron is located in the center which has three valence electrons and balances out the three chlorine. Here is structure of the compound Image courtesy- Google images -Ankit Kurmi. The shapes and bond angles of BeH2 BeCl2 CO2 AgNH32 BH3 BF3 BCl3 AlF3 COCl2 H2O H2S NH3 F2O PF3 PF5 PCl3 PCl5 H3O NCl3 CH4 CCl4 PCl4 PCl6- SF6 H3NBF3 NH3BF3 dot and cross diagrams bond angles H-B-H VSEPR molecule shape of BH3 bond angles H-C-H VSEPR molecule shape of CH3 bond angles F-B-F VSEPR molecule shape of BF3 bond angles Cl-B-Cl.
Check me out. For this molecule determine its a electronic geometry b number of nonbonding domains on the central atom and c polarity. Oral esophageal and stomach burns are common.
Structure properties spectra suppliers and links for. A trihalide of Boron BCl 3 consists of a single boron atom and three atoms of Chlorine. Molecular orbital diagrams give us an idea about the mixing of orbitals in molecules.
This is because the molecular arrangement of the chlorine atom is part of a complete triangular shape. Use VSEPR table to find the shape.
What Are The Hybridisation And Shape Of Bcl3 Quora

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
What Is The Structure Of Bcl3 Quora

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
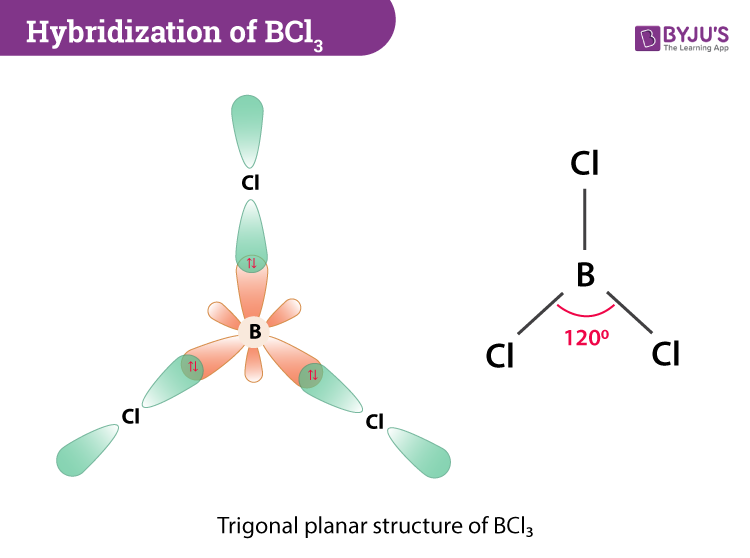
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape

Bcl3 Lewis Structure How To Draw The Lewis Structure For Bcl3 Youtube
0 Response to "Bcl3 Shape Diagram"
Post a Comment