Shape Of Bcl3 According To Vsepr Theory
The basic idea in molecular shapes is called valence shell electron pair repulsion VSEPR. The VSEPR theory is based on the assumption that the molecule will take a shape such that electronic repulsion in the valence shell of that atom is.
What Is The Structure Of Bcl3 Quora
A linear B trigonal planar C bent D tetrahedral E trigonal pyramidal.

Shape of bcl3 according to vsepr theory. Small moleculesmolecules with a single central atomhave shapes that can be easily predicted. The lone pair takes an equatorial position because it demands more space than the bonds. ClF 3 is a good illustration of this theory.
One arsenic trichloride molecule will have a total of 26 valence electrons - 5 from the arsenic atom and 7 from each of the three chlorine atoms. Hence the shape is trigonal bipyramidal. The VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule.
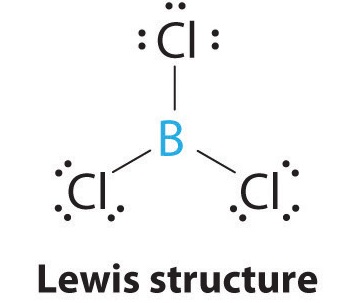
Use VSEPR table to find the shape. On the Lewis diagram identify the central atom. What is the molecular shape of BCl3 as predicted by the VSEPR theory.
This question hasnt been solved yet Ask an expert Ask an expert Ask an expert done loading. So the shape of BCl 3 molecule is trigonal planar. Hence the shape is tetrahedral.
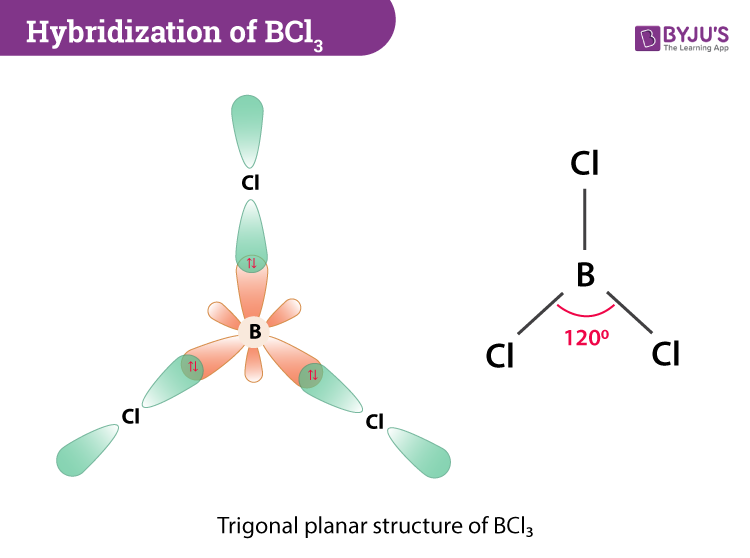
Boron forms three sp-p bonds with three chlorine atoms by using its half filled sp 2 hybrid orbitals. The electrons closest to the nuclei. We can see from the chart that BCl3 is an AX3 type molecule.
To predict the shape of the molecules first draw out the Lewis structure of the molecule. This VSEPR chart also gives us an idea about the hybridization of a molecule. It has 3 bonded atoms and 0 lone pairs.
Hence shape is triangular planar. Therefore the shape of the molecules are arranged so that the energy is minimized. The central atom Si has four bond pairs and no lone pair.
These are arranged in a trigonal bipyramidal shape with 102 F-S-F bond angles between the equatorial fluorine atoms and 173 between the axial fluorine atoms. To determine the molecular geometry of arsenic trichloride AsCl3 you must take a look at its Lewis structure. Download a copy of VSEPR shapes table here.
According to the chart the shape of this molecule is trigonal pyramidal. The shape according to VSEPR theory is given below MoleculeNumber of electron pairs around central atomMolecular geometryBond anglesBeC l2 2Linear180oBC l3 3trigonal planar120oS iC l4 4tetrahedral1095oAsF 5 5trigonal bipyramidalthree 120o two 90oH 2 S6non linearbent92oP H 3 5trigonal pyramidal935o. And thus the molecular geometry is trigonal planar.
Each chlorine atom uses its half filled p-orbital for the -bond formation. Molecules have shapes. Multiple Choice T-shape Trigonal pyramid Trigonal Planar Tetrahedral bent.
The central atom B has only three bond pairs and no lone pair. BeCl2 has minimum energy when it is a linear molecule. The Lewis structure of NF3 shows that its AX3E which has a trigonal pyramidal shape.
The bond angle will be about 107 degrees. Thus the shape of BCl 3 is trigonal planar with bond angles equal to 120 o. I1- Using VSEPR to Predict Shapes of Molecules The VSEPR predicted shapes of molecules can be found in a systematic way by using the number of electron pairs to determine the shape of the molecules.
The VSEPR chart is attached below. Inner shell electrons c. The central atoms As has five bond pairs and no lon pair.
3D shape also drawn hereC. The theory was first presented by Sidgwick and Powell in 1940. AX 3 has trigonal planar shape.
Valence Shell Electron Pair Repulsion Theory VSEPR is used to determine the shape and bond angle of a molecule. BCl3 takes the shape of trigonal planar. According to VSEPR theory molecules adjust their shapes to keep which of the following as far apart as possible.
Because electrons repel each other electrostatically the most stable arrangement of. Pairs of valence electronsThis answer is correct. According to VSEPR theory what is the shape of PCl3 based on the number of electron pairs around the central atom.
The answer is B trigonal pyramidal. The result is a. The standard application of VSEPR theory to this molecule is as follows.
Lesson Summary Molecule shapes can be predicted based on Lewis dot structure using the VSEPR theory. Apply VSEPR notation A X E ANumber of central atoms XNumber of surrounding atoms E Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 3 E 0. Valence shell electron pair repulsion theory is a simple way of rationalising the shapes of many compounds in which a main group element is surrounded by ligands.
Hence shape is linear. The arsenic atom will be bonded to the three chlorine atoms through single bonds that account for 6 of the. There is an abundance of experimental evidence to that effectfrom their physical properties to their chemical reactivity.
According to this model valence electrons in the Lewis structure form groups which may consist of a single bond a double bond a triple bond a lone pair of electrons or even a single unpaired electron which in the VSEPR model is counted as a lone pair.

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

How To Draw The Lewis Structure Of Bcl3 Boron Trichloride Youtube
Hybridization Of Bcl3 Hybridization Of Boron In Bcl3

Bcl3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Molecular Geometry Ppt Download

Using Vsepr To Determine Molecular Shape Bcl3 Intermolecular Forces Meristem Youtube
0 Response to "Shape Of Bcl3 According To Vsepr Theory"
Post a Comment