What Is The Electron Geometry Of Bcl3 Bcl3
This is because the molecular arrangement of the chlorine atom is part of a complete triangular shape. Draw the most appropriate Lewis structure s for BCl3.

Hybridization Of Bcl3 Hybridization Of Boron In Bcl3
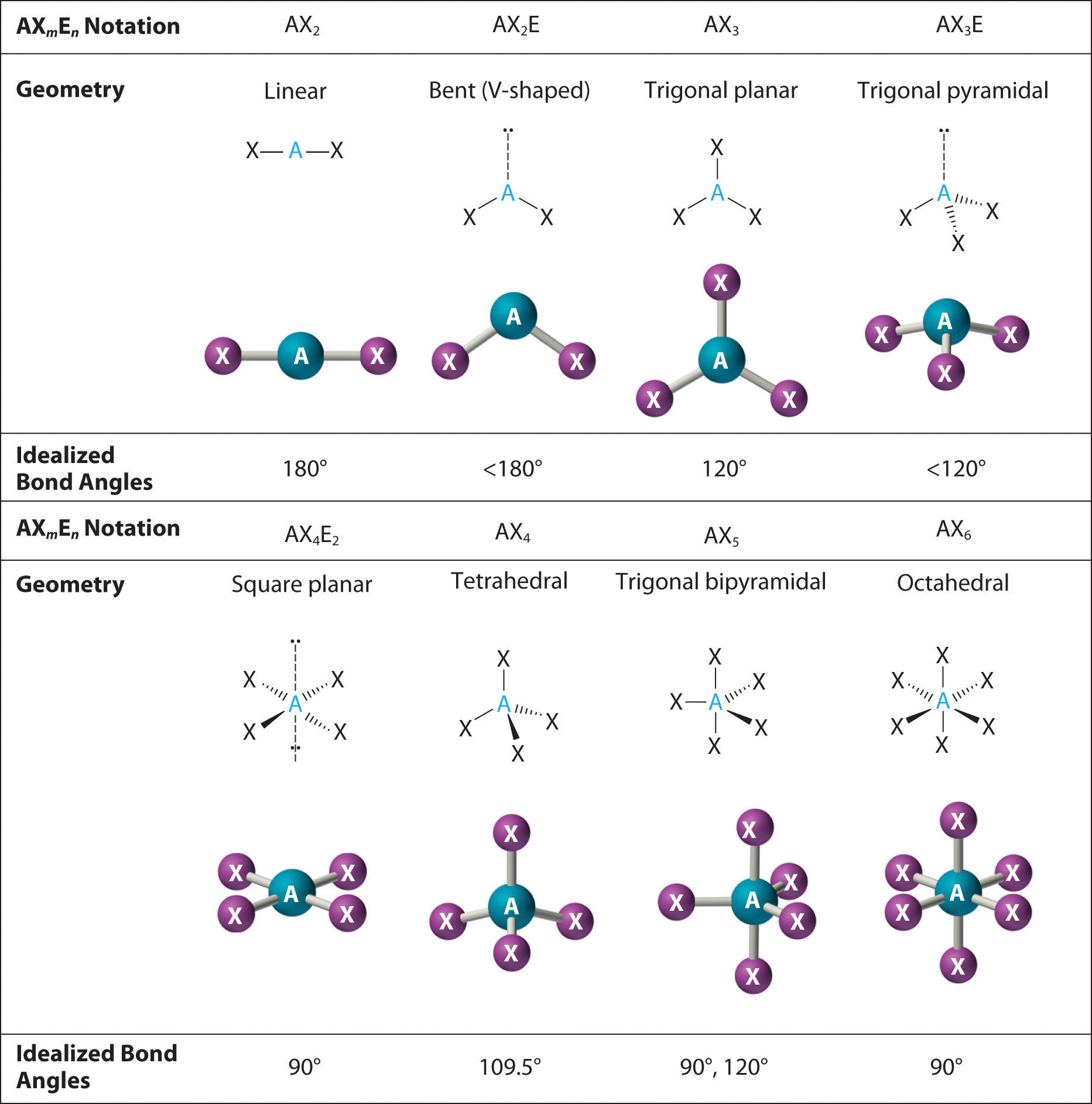
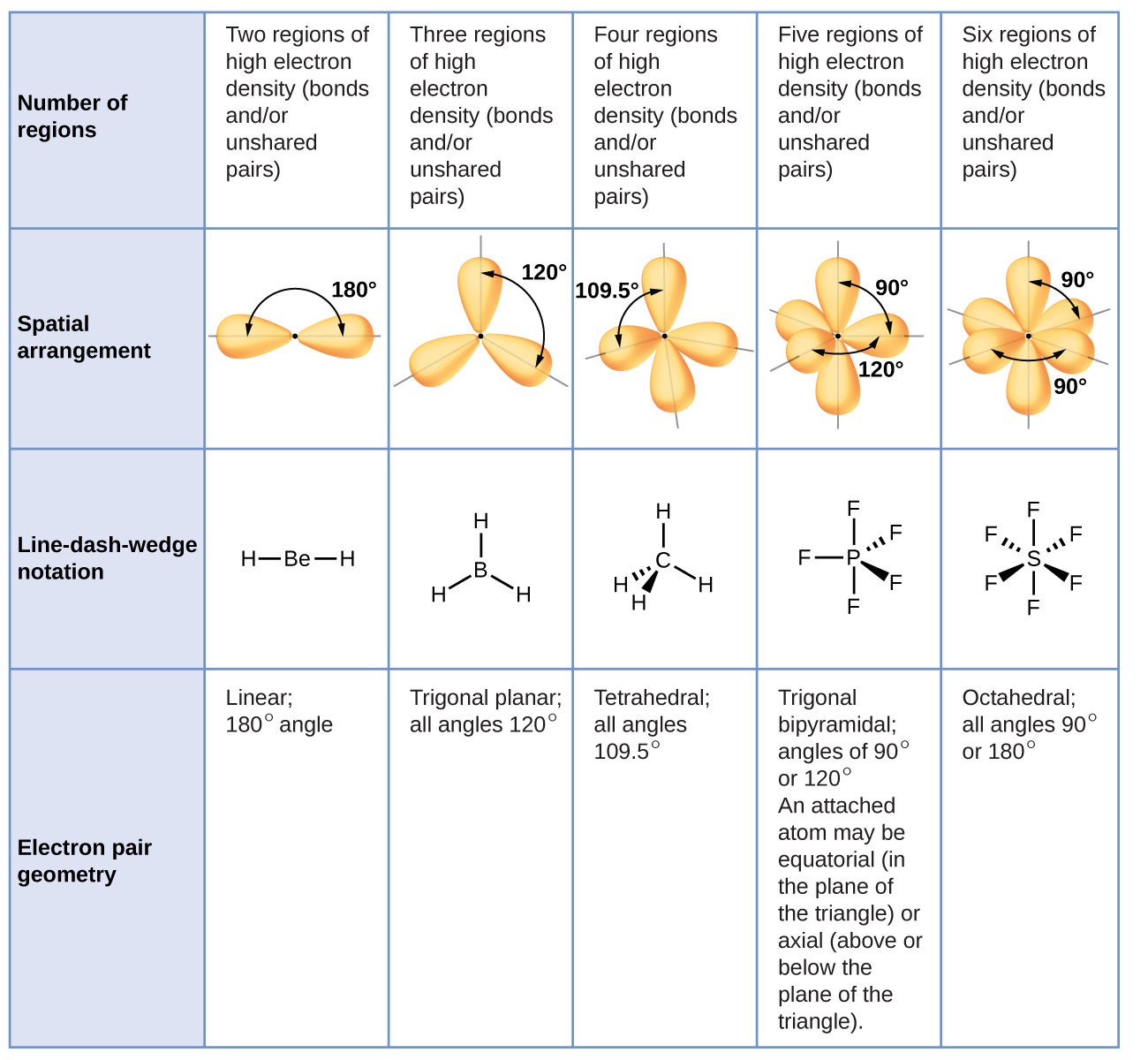
What is the electron domain geometry and the molecular geometry.
What is the electron geometry of bcl3 bcl3. Valence Shell Electron Pair Repulsion Theory VSEPR is used to determine the shape and bond angle of a molecule. The Correct Answer is 120 degreesFor the molecules in which there are no lone pairs of electrons on the central atom the electronic geometry is the same as the molecular geometry. Chemistry questions and answers.
Although the electronegativity difference between B and F is large 20 units BF3 is a covalent compound. There are lone pair s around the central atom so the geometry of BCl3 is. Describe the hybridization electron geometry molecular geometry and polarity for each and discuss similarities and differences.
Recall that for predicting geometry double and triple bonds count as only one electron pair. BF3 has trigonal planar molecular geometry b. The electron-pair geometry around the C atom in CS2 is.
Our mission is to help you succeed in your Chemistry class. Both BCl3 and ICl3 have 3 bonds. ClF3 has T-shaped geometry.
The electron-pair geometry around the B atom in BCl3 is. Lone Pairs Single or multiple bonds around the central atom 3. Lone Pairs around central atom 0.
All three bond angles in BF3 are 120 d. Does the molecule have a dipole. This gives us 21 valence electrons for 3 Chlorine atoms.
Boron forms 3 sp-p bonds with three chlorine atoms. The bond angle is 120 o. Total Valence Electrons in BCl 3 3 from Boron 7 x 3 from Chlorine 24 Valence Electrons Thus BCl 3 has 24 total valence electrons.
What is the hybridization of the central atom. BF3 has trigonal pyramidal electronic geometry c. The central atom also has a symmetric charge around it and the molecule is non-polar.
Therefore from the above relation 1 the total number of valence electrons in BCl 3 is given by. The central boron atom in boron trichloride BCl3 is electron-deficient enabling the molecule to accept additional pairs of electrons and act as a Lewis Acid. Clutch really helped me by reinforcing the.
The B atom does not satisfy the octet rule e. Note that Boron can have a full outershell with only six valence electrons. If we look at the structure BCl 3 molecular geometry is trigonal planar.
The molecular geometry of the BeCl2 molecule is The molecular geometry of the CHF3 molecule is When in the presence of a strong acid a water As shown below an electron carrier such as PCl5 has _____ electron domains and a The information carried by a DNA molecule is in _____. Although the electronegativity difference between B and F is large 20 units BF3 is a covalent compound. In the Lewis structure for BCl3 the central atom Boron will only have six valence electrons.
1 The Molecular Geometry Of The BCl3 Molecule Is And This Molecule Is - A Trigonal Pyramidal Polar B Trigonal Pyramidal Nonpolar C Trigonal Planar Polar D Trigonal Planar Nonpolar E Trigonal Bipyramidal Polar 2 Which One Of The. What is the value of the bond angles in BCl3. The Lewis diagram for BCl3 is.
I hope that this blog post helps you understand all the aspects of this molecule in depth. Let us know in the comments below which other molecules Lewis structure you would like to learn. BCl3 Molecular Geometry According to VSEPR theory the molecular geometry of boron trichloride is trigonal planar with a bond angle of 120 degrees.
The Lewis Acid-base theory defines acids as species accepting pairs of electrons. BCl3 Molecular Geometry The molecular geometry of BCl3 is trigonal pyramidal. Each sp 2 hybrid orbitals will have an unpaired electron.
BF3 has trigonal pyramidal electronic geometry c. 1 The Molecular Geometry Of The BCl3 Molecule Is And This Molecule Is - A Trigonal Pyramidal. BCl 3 Molecular Geometry And Bond Angles.
The B atom does not satisfy the octet rule e. The Lewis diagram for CS2 is. The molecular geometry of a molecule describes the three-dimensional shape of just the atoms.
BF3 has trigonal planar molecular geometry. All three bond angles in BF3 are 120 d. The boron is located in the center which has three valence electrons and balances out the three chlorine.
It has a tetrahedral electron geometry and trigonal pyramidal shape. How many o and bonds are there. BCl3 is a trigonal planar molecule like the other boron trihalides and has a bond length of 175pm.
Bcl3 Lewis Structure Molecular Geometry Hybridization And Shape

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have

Bcl3 Boron Trichloride Molecular Geometry Bond Angles And Electron Geometry Youtube
10 3 Vsepr Geometry Chemistry Libretexts

Bcl3 Lewis Structure And Molecular Geometry Youtube
Bcl3 Boron Trichloride Molecular Geometry Bond Angles And Electron Geometry Youtube


0 Response to "What Is The Electron Geometry Of Bcl3 Bcl3"
Post a Comment